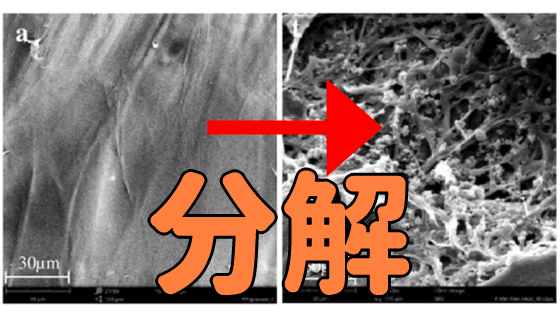
A way to easily and safely destroy ``eternal chemicals'' used in frying pans and shampoos is discovered

Simple mix of soap and solvent could help destroy 'forever chemicals' | Science | AAAS
https://www.science.org/content/article/simple-mix-soap-and-solvent-could-help-destroy-forever-chemicals
Scientists find simple, safe methods to destroy 'forever chemicals'
https://www.france24.com/en/live-news/20220818-scientists-find-simple-safe-method-to-destroy-forever-chemicals
The reason why PFAS is difficult to decompose is that the PFAS molecule contains a carbon-fluorine covalent bond . Fluorine has the highest electronegativity , so its covalent bond with carbon is one of the strongest chemical bonds. PFAS is a compound in which this carbon-fluorine covalent bond is long like a chain.
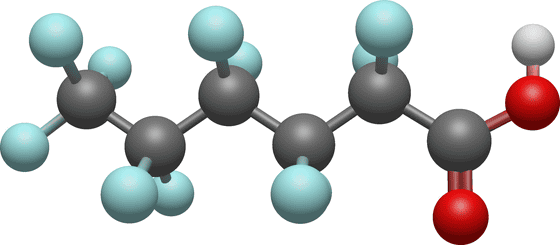
However, according to the research team, the carboxy group (R-COOH) of some PFAS is a weak point. When PFAS was mixed with a mixture of
The research team reports that what is left after the decomposition of PFAS is easy-to-recover fluorine ions and harmless compounds containing carbon and oxygen. The cost of DMSO and sodium hydroxide is low, and the relatively low temperature makes processing easy and safe. Therefore, it has been suggested that it may become possible to preliminarily decompose and remove PFAS from drinking water with simple equipment.
However, although there are 12,000 types of PFAS registered with the US Environmental Protection Agency, PFAS with carboxyl groups account for about 40% of the total. The research team states that this 40% PFAS may be degraded using DMSO, but has not been tested. Also, PFAS used in flame retardants and batteries has a sulfone group (R-SO 3 H) instead of a carboxy group, so it is not decomposed by DMSO.
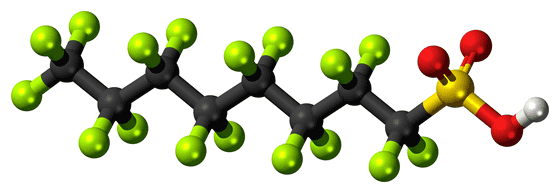
The research team said, 'Every molecule has its own weaknesses.
Related Posts:
in Science, Posted by log1i_yk