Does the 'phenomenon of water droplets floating on a hot iron plate' occur with liquids other than water?

When water drops are dropped on a heated frying pan, the water evaporates momentarily at the part in contact with the frying pan to form a film of water vapor, and the phenomenon that the water drops move smoothly as if floating on the frying pan is the '
Phys. Rev. Lett. 127, 204501 (2021) --Triple Leidenfrost Effect: Preventing Coalescence of Drops on a Hot Plate
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.127.204501
Amazing Video Reveals a New Kind of Leidenfrost Effect We've Never Seen Before
https://www.sciencealert.com/we-ve-just-discovered-a-new-kind-of-leidenfrost-effect
The research team led by Mr. Vasquez dropped liquids such as water, ethanol, methanol, chloroform, and formamide onto a metal plate heated to 250 degrees Celsius and observed the behavior. As a result, it was found that not only the Leidenfrost effect can be confirmed in other than water, but also that droplets with similar characteristics coalesce and droplets with large differences in characteristics repel each other.
If you actually drop water and ethanol on a metal plate, you can see how each drop behaves by watching the following movie.
The triple Leidenfrost effect in water and ethanol --YouTube
The large droplets are water, and the small droplets colored with blue dye are ethanol.

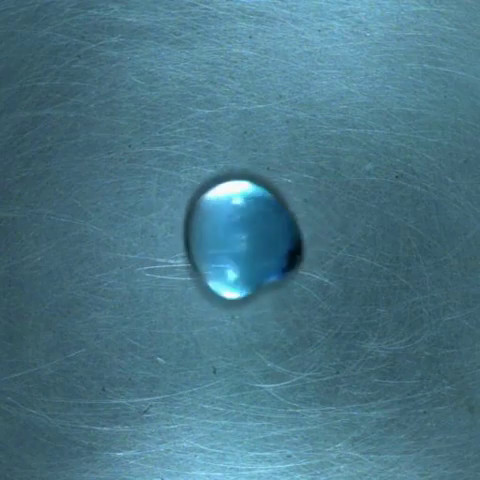
The two droplets behave as if they collide and repel each other.
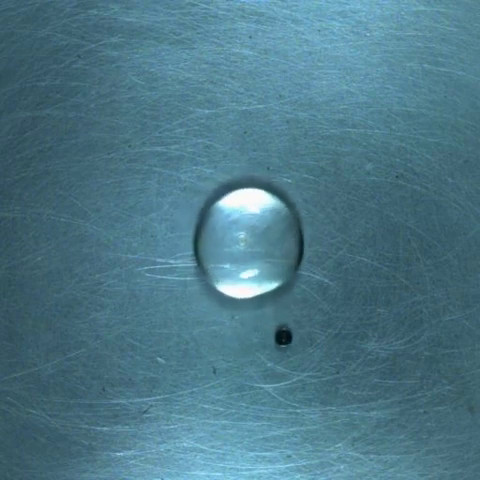
After several collisions, the two droplets merge.
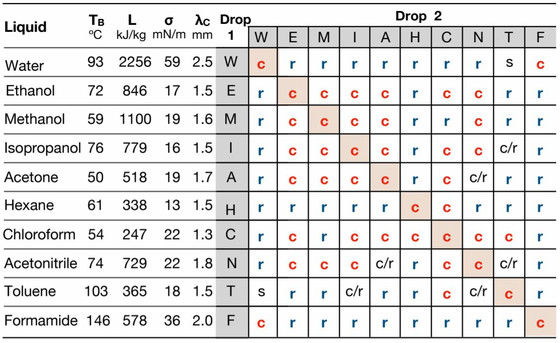
The table below shows water (W), ethanol (E), methanol (M), isopropanol (I), acetone (A), hexane (H), chloroform (C), acetonitrile (N), toluene (T), A summary of the results of experiments with droplets of formamide (F). 'C' indicates the coalescence of the droplets, 'r' indicates the repulsion of the droplets, and 's' indicates that they remained separated without mixing.
The Leidenfrost effect occurs when the liquid evaporates at the contact surface between the hot surface and the droplets, such as an iron plate, and a layer of vapor prevents the liquid from coming into contact with the hot surface. The research team's experiments suggest that a layer of vapor is generated not only on the contact area between the droplet and the hot surface, but also on the surface of the droplet, which may cause the two droplets to repel each other without mixing. increase.
The research team said, 'The repulsion between these droplets is a phenomenon that occurs because the boiling points of the two droplets are different, and it occurs when the high boiling point droplet acts as a high temperature surface for the low boiling point droplet. We show that three contact areas are generated at the same time: the surface of the high boiling point droplet, the contact surface of the high boiling point droplet, and the contact surface of the low boiling point droplet. We call the phenomenon the 'triple Leiden Frost effect.' '
Related Posts:
in Science, Posted by log1i_yk






