"Burning ice" A new mechanism to form methane hydrate is discovered

"Burning ice" that has an appearance like white ice and ignites and burns when bringing the fire closermethane hydrateIs asleep in a state of being adhered to sediments in the sea at low temperature and high pressure. There was a mystery part about how the methane hydrate was formed on the whole but the details of it have been clarified from the latest research.
Effect of Electric Field on Gas Hydrate Nucleation Kinetics: Evidence for the Enhanced Kinetics of Hydrate Nucleation by Negatively Charged Clay Surfaces - Environmental Science & Technology (ACS Publications)
https://pubs.acs.org/doi/10.1021/acs.est.7b05477
Burning ice in oceanic clay rich sediment - ScienceDaily
https://www.sciencedaily.com/releases/2018/04/180409103858.htm
KAIST disclose the formation of burning ice in oceanic clay rich sediment | EurekAlert! Science News
https://www.eurekalert.org/pub_releases/2018-04/tkai-kdt040918.php
This research was conducted at the National University of KoreaKAIST(Korea Advanced Institute of Science and Technology) research team. Professor Kwon Tae-hyeok and the research team of the Department of Civil and Environmental Engineering discovered that clay minerals in clay-rich oceanic deposits promote the formation of "gas hydrate" containing methane hydrate, We advocated the principle.
Methane hydrate is a type of gas hydrate in which methane molecules are stably fixed in a three-dimensional network structure of water molecules, and it exists at the bottom of the world. Among them, we know that the world's leading methane hydrate vein exists around Nankai Trough in western Japan. The content ratio of methane and water is about 15:85 and it has a burning characteristic when putting on fire and half of carbon dioxide emissions compared with oil and coal so it is promising as an alternative fuel for global warming countermeasures It is an energy source being.

ByNathan Harper
So far, the formation of gas hydrate has been considered only in clay sediments. However, a new discovery that gas hydrate was generated in the marine sediments resulted in a new problem being raised in the mechanism of its formation.
Since the surface of natural clay mineral is negatively charged, physicochemical interaction between clay and water inevitably occurs. Such an interaction plays an important role in the generation of natural gas hydrate in clay-rich sedimentary layers.
However, there was a situation that it was experimentally difficult to analyze the formation of gas hydrate because cations existed in the clay molecules and charge balanced on the surface. Therefore, clay particles inevitably release cations when mixed with water, interpretation of experimental results was complicated.
To overcome this limit, the research team polarized the water molecules by electric field and monitored the induction time of the water molecules forming the gas hydrate. Then, we found that under certain circumstances an electric field of 10 kV / m causes nucleation of gas hydrate. This is due to partial destruction of hydrogen clusters to which hydrogen is bonded and thermal energy, which is the opposite result to the conventionally conceived "nucleation speed reduction".
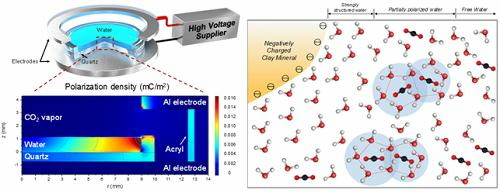
According to Mr. Kwon, "This research gave us a deeper insight into the generation of gas hydrate in clay-rich marine sediments, and in the near future we will start commercializing methane gas from natural gas hydrate deposits We will be able to produce it in the future. "
Related Posts:
in Science, Posted by darkhorse_log