"Periodic Table of the Elements, in Pictures and Words" showing by illustration what elements of the periodic table are used
Speaking of the "periodic table" in which elements are arranged in atomic number order, there are many people who have experienced rote memorizing with "militia Ribe my ship ......" mysterious spells. However, it is such a tasteless and uninteresting thing, Illustration showing what purpose each element is used in the periodic table which was not interested in the student days, info in which simple feature explanation was added graphic"The Periodic Table of the Elements, in Pictures and WordsIf you look at "chemistry interests may come of age.
Elements.wlonk.com
http://elements.wlonk.com/ElementsTable.htm
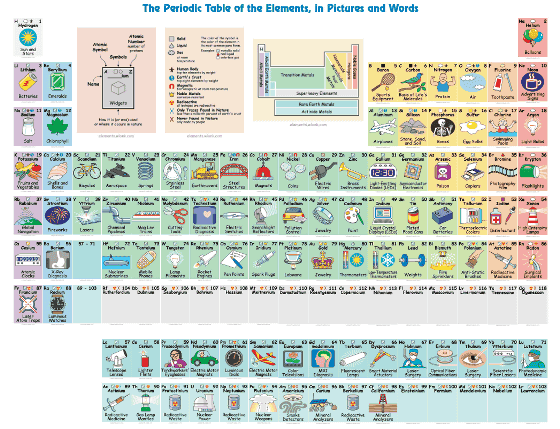
This is the periodic table with illustration & explanation "The Periodic Table of the Elements, in Pictures and Words". When you hit the mouse cursor on each element's card, the explanation card is displayed for 3 pieces in the margin at the top of the periodic table.
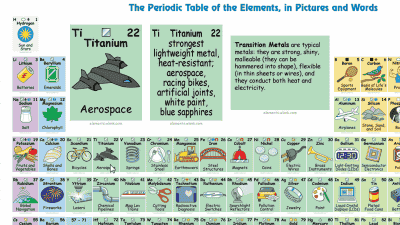
Explanation In addition to elemental symbols and atomic numbers, card 1 explains what kind of use the element is used with illustration.
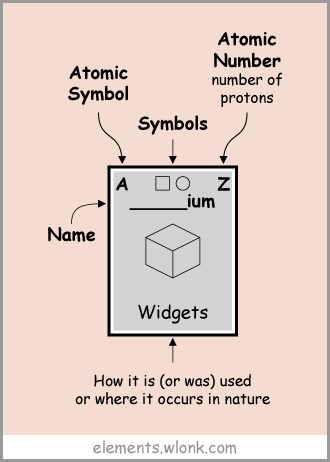
Explanation The explanation of the icon displayed at the top of the card 1 is as follows. Solid / liquid / gas, constituent elements of the human body, presence / absence of magnetism, presence of radioactivity, etc. are indicated by icons.

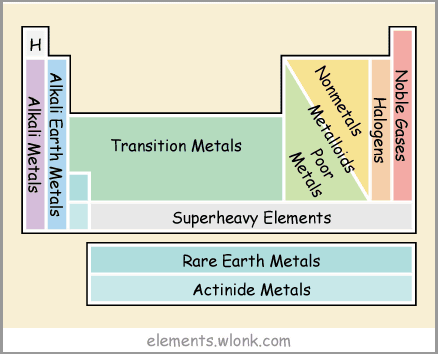
Groups of different properties such as alkali metals, alkaline earth metals, noble gases, transition metals and rare earths are also displayed.
Atomic number "1" "hydrogen(H) ", a description was displayed on the card. Hydrogen is a constituent element of "Sun and Stars (sun and star)" and it is said to be 90% of the material of the universe. It belongs to Group 1 of the periodic table, but is an element of independent existence which is not classified as alkali metal.

Next, the atomic number "2" "helium(He) ". Helium is the second lightest gas after hydrogen, which is used for balloons. It is a very stable gas which does not react with other atoms because it is closed shell with 8 outermost shell electrons.

Atomic number "3"lithium(Li) "is a metal often used for batteries. Lithium, which is the lightest metal, is an alkali metal that is highly responsive, so it does not exist as a single body in nature. It floats on water and it has the characteristic of "burning" while sparking.

The atomic number "8" "oxygen(O) "is a gas that contains as much as 21% in the air.Clarke numberIs about 50, which is the most abundant element near the surface of the earth.

Atomic number "13"aluminum(Al) "is used for aircraft materials. Although it is lighter and better in workability, it will become brittle as it corrodes.

At atomic number "53"Iodine(I) "is contained in disinfectant solution. Iodine dissolved in liquid has a deep purple color and it is effective for wound disinfection and throat disease. Iodine has high reactivity, and it reacts even with alkali metal to produce salt.

Atomic number "56"barium(Ba) "is a metal familiar with X-ray examination. Pure barium has very soft and brittle properties.

The atomic number "79" "Money(Au) "is used for jewelry. Metal gold with color can be gold leaf or plated with malleability. There is also a characteristic that the conductivity of heat and electricity is high.

Atomic number "92"uranium(U) "is used as nuclear fuel in nuclear power plants.

The atomic number "55"cesium(Cs)Atomic clockAnd that it is used for GPS.

The atomic number "70"ytterbium(Yb) is used for fiber laser.scandiumYayttriumThe chemical properties are similar and it is difficult to separate.

By looking at "The Periodic Table of the Elements, in Pictures and Words" which explains the characteristics of elements with illustration and simple explanation, it seems that you can learn familiar elements as well as elements that are not so enjoyable.
Related Posts: