The development of the world's first safe and efficient water-based aluminum-ion battery reaches the first stage

Most batteries contain some kind of toxic substance and disposal can pollute the environment. A team from Flinders University in South Australia and Zhejiang University of Science and Technology in China published a report on the first stage of development of a non-toxic, safe and efficient ' aqueous aluminum ion battery ' in the journal of the American Chemical Society. Did.
Lewis Acid-Induced Reversible Disproportionation of TEMPO Enables Aqueous Aluminum Radical Batteries | Journal of the American Chemical Society
'Radical' new green power source – News
Many batteries contain substances such as lead, cadmium, and mercury that are toxic to humans and animals and contaminate soil and water. It is also a problem that these harmful substances remain in the environment for a long time.
2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO), which is sometimes used as a positive electrode material for polyvalent metal ion batteries, features a high redox potential and a fast electrochemical reaction rate. . However, TEMPO and its derivatives are not commonly used in newer aluminum-ion batteries due to their known
Zhongfan Jia, associate professor at Flinders University, said, 'Polyvalent metal-ion batteries, including Al³⁺, Zn²⁺, and Mg²⁺, use the abundant elements found in the Earth's crust to produce much higher energy densities than lithium-ion batteries. 'Aluminium-ion batteries, especially those based on the third most abundant element, alninium, have the potential to become sustainable, low-cost energy storage systems.'
However, aluminum ion batteries have the problem that the movement of Al³⁺ ion complexes is slow and the cathode efficiency is low.
Here, the research team investigated the electrochemical behavior of TEMPO in organic and aqueous Lewis acid electrolytes. We have clarified for the first time the irreversible disproportionation of TEMPO in organic electrolytes. We also found that reversible electrochemical redox reactions are possible across TEMPO in aqueous Lewis electrolytes.
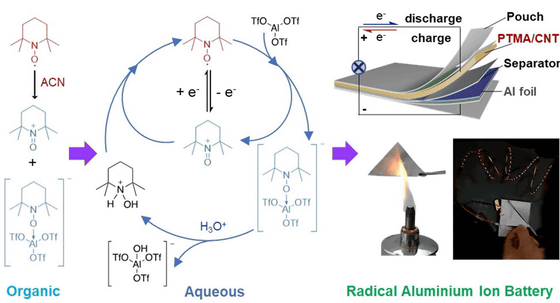
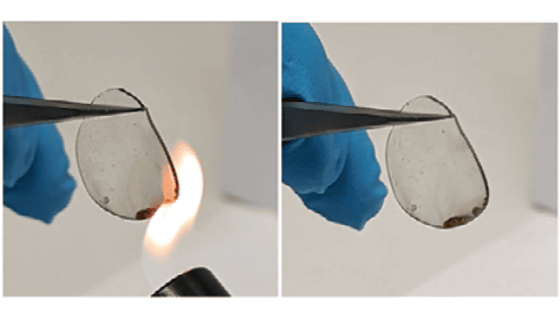
Based on these findings, the research team designed a stable radical polymer water-based aluminum-ion battery that is fire-resistant and air-stable. A stable output of 1.25V was achieved for 800 cycles with a capacity of 110mAh per gram of material. The loss per cycle was only 0.028%.
This work shows the promise of using non-conjugated organic electroactive materials in aluminum-ion batteries that rely on conjugated organic molecules.
Associate Professor Jia hopes to develop a soft pack battery using biodegradable materials in the future and make the battery safe and sustainable.
Related Posts:
in Science, Posted by logc_nt