The US Food and Drug Administration approves the first prostate cancer detection AI 'Paige Prostate', improving the cancer detection rate by doctors to about 97%
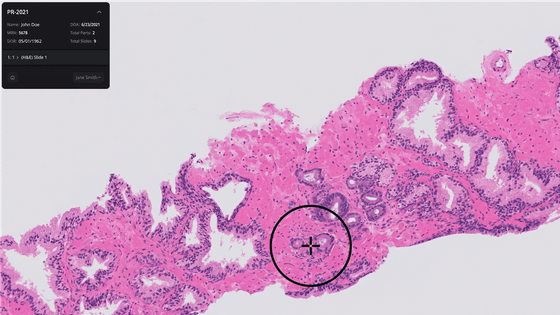
On September 21, 2021, the U.S. Food and Drug Administration (FDA) approved AI's Paige Prostate, designed to identify cancer from prostate scans, and launched it as software to assist specialists. Announced that it was allowed. This is expected to greatly improve the number
FDA Authorizes Software that Can Help Identify Prostate Cancer | FDA
https://www.fda.gov/news-events/press-announcements/fda-authorizes-software-can-help-identify-prostate-cancer
Paige Receives First Ever FDA Approval for AI Product in Digital Pathology | Business Wire
https://www.businesswire.com/news/home/20210922005369/en/Paige-Receives-First-Ever-FDA-Approval-for-AI-Product-in-Digital-Pathology
Prostate cancer, which occurs in the prostate gland, which is the male genital organ, is the most common cancer in American men except for non-melanoma skin cancer, and is the leading cause of death in men who die from cancer.

Paige, an AI company founded in 2017, announced on September 21 that its prostate cancer detection AI, Paige Prostate, was submitted by the FDA through a
Upon approval, the FDA evaluated the results of a clinical trial involving 16 specialists. A total of 527 slides were used in the study, including 171 slides of prostate biopsy with malignant tumor (cancer) and 356 benign slides, with each specialist assisting Paige Prostate. We analyzed with 'yes' and 'without assistance'.
Testing showed that Paige Prostate improved cancer detection rates from 89.5% to 96.8% by about 7 percentage points, but did not affect benign tumor detection rates. In addition, Paige Prostate-backed physicians had a 70% reduction in false-negative diagnoses and a 24% reduction in false-positive diagnoses, and these effects depended on the specialist's experience and whether the test was remote. It wasn't.

'Pathologists perform daily biopsies of suspected tissues such as prostate cancer,' said Tim Stenzel, director of in vitro diagnosis and radiation health at the FDA's Medical Devices and Radiation Health Center. The AI's identification of areas of suspicion of cancer on biopsy scans will help pathologists make diagnoses that lead to appropriate treatment. '
'Paige Prostate's approval by the FDA means a new era of computer-aided diagnosis in pathology has begun,' said Dr. David Crimstra, MD, co-founder and chief medical officer of Paige. '.
Related Posts: