Introducing an epoch-making technology that greatly improves the performance of cryo-electron microscopy, which will be used to clarify the three-dimensional structure of proteins

Proteins, which are chain polymer compounds in which 20 or more kinds of amino acids are linked, are involved in almost all functions performed in the human body, and investigating the structure of proteins involves the development of drugs and the treatment of diseases. It is very important for studying the mechanism. In May 2020, several research teams succeeded in improving a method called '
Breaking the next Cryo-EM resolution barrier – Atomic resolution determination of proteins! | bioRxiv
https://www.biorxiv.org/content/10.1101/2020.05.21.106740v1
Single-particle cryo-EM at atomic resolution | bioRxiv
https://www.biorxiv.org/content/10.1101/2020.05.22.110189v1
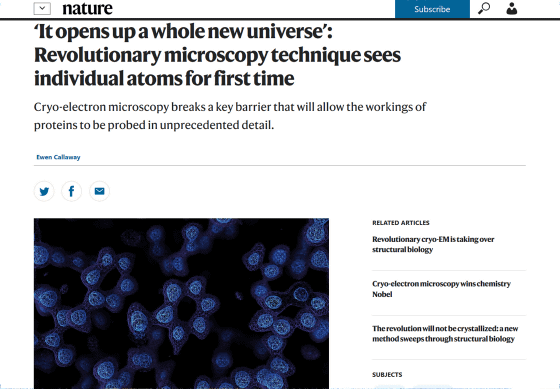
'It opens up a whole new universe': Revolutionary microscopy technique sees individual atoms for first time
https://www.nature.com/articles/d41586-020-01658-1
Many proteins in the body perform various functions by interacting with each other, and proteins are the cause of diseases and the targets of drugs. However, although the way
Cryogenic electron microscopy fixes a sample by freezing it at a low temperature, and then analyzes it to make the structure of the sample closer to that in the living body compared to ordinary electron microscopy in which the sample is prepared by staining or chemical fixation. It is believed to be observable. It also has the advantage of avoiding damage to the sample due to electron beams and high vacuum.
Since around 2013, the 'resolution revolution' of cryo-electron microscopy has been promoted by the development of electron detection technology and image analysis software. With this progress, cryo-electron microscopy can observe protein structures with almost the same accuracy as X-ray crystallography. However, cryo-electron microscopy has not been able to perform structural analysis with atomic resolution up to now, and in order to understand the structure of proteins with atomic resolution, we still have to rely on traditional X-ray crystallography. It was.
However, the process of crystallizing the protein required for X-ray crystal structure analysis may take several months to several years, and many of the medically important proteins form crystals that can be used for X-ray crystal structure analysis. I don't. On the other hand, cryo-electron microscopy allows analysis if the protein is in a purified solution, so it is expected that research will proceed more smoothly if atomic resolution can be achieved.

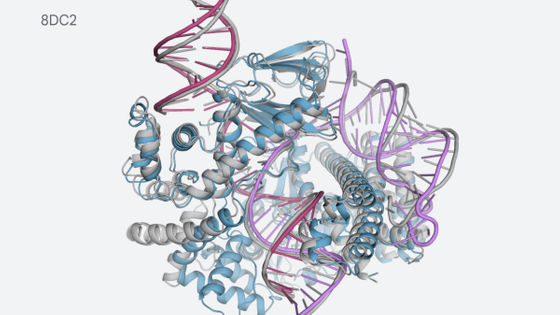
In May 2020, two independent research teams announced a method to improve the resolution of cryo-electron microscopy, and reported that they achieved 'atomic resolution' to identify the atoms that make up proteins. In order to improve the performance of cryo-electron microscopy to atomic resolution, the two research teams conducted research targeting a protein called
One study, led by Holger Stark of the Max Planck Institute for Biophysical Chemistry , improved the resolution by using a device that kept the velocity of the electrons reaching the sample constant, achieving a resolution of 1.25 angstroms , comparable to atomic resolution. Achieved
Another study, led by Sjors Scheres and Radu Aricescu of the MRC Institute for Molecular Biology , uses another technique that keeps electrons moving, while reducing noise after the electrons leave the sample. We have achieved a resolution of 1.2 angstroms by using this technology and a more sensitive electronic detection camera. Scherses says that the resolution of 1.2 Å is enough to distinguish hydrogen atoms from proteins and water molecules around them.
Scheres and colleagues also tested a simplified form of the GABAA receptor protein, which targets many drugs such as general anesthesia and anxiolytics. By using a new method, the research team was able to achieve 1.7 angstrom resolution even with GABAA receptor, and although it was not possible to achieve atomic resolution, it was possible to know details that could not be known so far.
``We all know that the Max Planck and MRC Molecular Biology groups are performing at a phenomenal level of performance,'' said Danev Radostin of the University of Tokyo, an expert in cryo-electron microscopy. I am very excited and amazed.' ' True'atomic resolution' is a real milestone,' said Professor John Rubinstein of the University of Toronto.
Scheres argues that his findings are likely to consolidate the position of cryo-electron microscopy as a major tool for studying protein structure, but Stark is unique to X-ray crystallography. Pointed out that it has merit. If it is known that the target protein is large and can be crystallized, it is easy to identify the protein structure in the state of being bound with a potential drug. “Each method still has its strengths and weaknesses,” he said.
Related Posts:
in Science, Posted by log1h_ik