How advanced is vaccine development for new coronavirus infections?
With the release of the new coronavirus (SARS-CoV-2)
The COVID-19 vaccine development landscape
https://www.nature.com/articles/d41573-020-00073-5
As of April 8, 2020, there are 115 global R & D projects for COVID-19 vaccines, 78 of which are in progress, and 37 of which are based on public information or proprietary sources to determine development status It is in a state that can not be done. Of the 78 projects in progress, 73 are still in the research or pre-clinical phase.
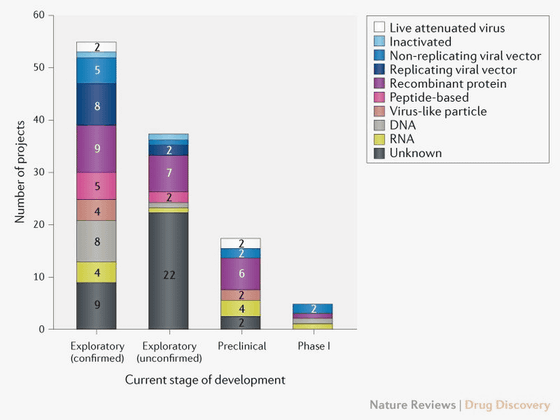
Vaccine projects progressing to the clinical development stage include
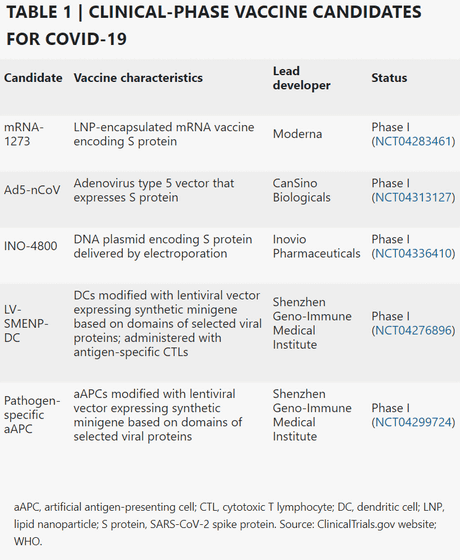
Of these, mRNA-1273 was the first to reach the clinical stage, and on March 16, 2020, it was reported that the world's first human clinical trial was conducted as a COVID-19 vaccine.
Vaccine development projects are being conducted in a total of 19 countries. Most of the COVID-19 vaccine development activity is in North America, with 36 vaccine development organizations confirmed to be in progress. There are 14 companies in China, 14 in Asia and Australia excluding China, and 14 in Europe. China, which is considered the center of COVID-19 outbreak, is particularly active in vaccine development and plans to start additional vaccine development projects.
However, because the nature of COVID-19 may vary from region to region, analysts say that vaccine research and development should be conducted in Africa and South America to effectively control pandemics. However, research facilities of SARS-CoV-2 in Africa has been misunderstood as 'sprinkle the disease', the incident that is destroyed in response to the attack to local residents also generated has been, if not the understanding of local residents It may not be slow.
Most of the vaccines under development use the protruding protein (S protein) on the surface of SARS-CoV-2 as an antigen to cause an immune response. For example, mRNA-1273, which began clinical trials just two months after the identification of the SARS-CoV-2 gene sequence, expresses the S protein based on mRNA (messenger RNA), as the name suggests, and then uses the antibody as an antibody. It seems to be a mechanism to make.
Also, some vaccines are planning to be effective at lower doses by adding vaccine adjuvants called adjuvants . However, since all projects are still in development, the information available is limited and details on how each vaccine produces antibodies are not clear.
The analyst team said that the development of the COVID-19 vaccine is taking place at a much faster pace than the relatively fast paced development of the Ebola vaccine , `` Vaccines in response to the COVID-19 pandemic. The global R & D is progressing at an unprecedented scale and speed, 'he said, adding that vaccines may be available by early 2021. 'To ensure that the COVID-19 vaccine is provided equitably around the world, especially in resource-poor areas, vaccine developers, regulators, policy makers, funders, public health agencies, We need strong international coordination and strength. '
Related Posts:
in Science, Posted by log1i_yk