Succeeded in creating the world's first `` ring composed only of carbon atoms ''

by
Carbon atoms bind to each other in a variety of ways and shapes, and numerous allotropes such as carbon nanotubes , diamond , and graphite have been identified. Harvard and IBM research teams will create a `` ring composed of carbon atoms '' that many chemists have failed to make even though it has been pointed out that it may exist as an allotrope of carbon Succeeded.
An sp-hybridized molecular carbon allotrope, cyclo [18] carbon | Science
https://science.sciencemag.org/content/early/2019/08/14/science.aay1914
Chemists make first-ever ring of pure carbon
https://www.nature.com/articles/d41586-019-02473-z
In carbon nanotubes, one atom is bonded to three atoms, whereas in diamond, atoms are bonded to four atoms. Carbon atoms can be chemically bonded to other atoms in various configurations. In addition, since carbon atoms can be bonded to only two nearby atoms, the combination of carbon atoms on both sides can be made cyclic to create a “cyclic ring made of only carbon atoms. It has been said that a “carbon allotrope” should be created.
This ring of carbon atoms is called “ Cyclocarbon ” and many chemists have tried to synthesize it, but the attempts were unsuccessful. The ring structure is more chemically reactive than diamond or graphene , and is less stable when bent.
by
The research team of Przemyslaw Gawel and Lorel Scriven , chemists at Oxford University, has continued research to create such cyclocarbon. The research team first created a ring containing not only carbon atoms but also oxygen atoms, sent the rings to IBM's laboratory in Switzerland, and tried to remove the oxygen atoms from the ring with the laboratory equipment That's right.
The Swiss research team, a collaborator, placed a ring sent from Oxford University on the sodium chloride layer in a vacuum chamber and used an electric current to remove oxygen atoms from the ring. After trial and error, the research team succeeded in creating a carbon-only ring, namely cyclocarbon. The completed Cyclocarbon seems to have a structure with 18 carbon atoms, and Mr. Scriven commented, 'I never thought I could see this.'
IBM researchers investigated Cyclocarbon under a microscope and found that the carbon ring had alternating single and triple bonds. The theory of how the cyclocarbon is bonded has not been consistent so far, but it has finally been settled by actually creating it.
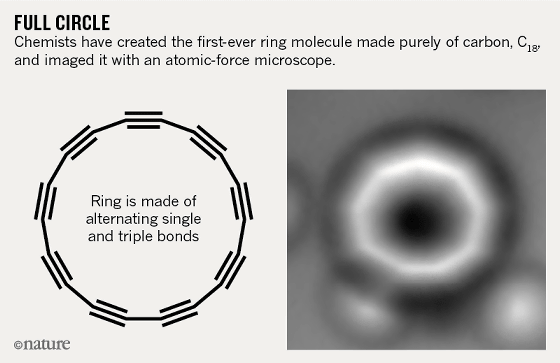
Since the structure in which single bonds and triple bonds alternate gives Cyclocarbon semiconductor properties, it seems that it may be applicable to molecular-sized electronic components in the future using the properties of Cyclocarbon. Gawel says he will continue to investigate the properties of cyclocarbon in the future, and plans to conduct research on how to mass-produce cyclocarbon.
Professor Yoshito Tobe , a scientist at Osaka University, said, “Many chemists including me have tried to determine the molecular structure by capturing cyclocarbon, but it was a waste.” Praised the results of the research team.
Related Posts:
in Science, Posted by log1h_ik