Succeeded in producing a catalyst that suppresses charge and discharge deterioration of "air zinc battery" which is higher performance and less expensive than lithium ion battery with ordinary metal
It extracts electric power by reacting "common zinc" of common metals with "oxygen" in the airZinc air battery"Is lightweight, inexpensive, high energy density, batteries that are all three times, but because it is not suitable for charging, its application was limited. Australian researchers have succeeded in generating catalysts that minimize deterioration of air zinc batteries by charging and discharging with ordinary inexpensive metals such as iron and nickel.
Amorphous Bimetallic Oxide-Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries - Wei - 2017 - Advanced Materials - Wiley Online Library
http://onlinelibrary.wiley.com/doi/10.1002/adma.201701410/abstract
University of Sydney charges ahead on zinc-air batteries - The University of Sydney
http://sydney.edu.au/news-opinion/news/2017/08/15/university-of-sydney-charges-ahead-on-zinc-air-batteries.html
An air zinc battery using oxygen for the positive electrode and zinc for the negative electrode is a battery having a theoretical energy density as high as 1.3 kWh / kg, and from a high energy density of about twice that of the lithium ion battery which has been put to practical use, a lithium ion battery It is expected as an alternative battery. In addition to high energy density, oxygen in the air is used for the positive electrode, so the positive electrode active material is unnecessary, which is advantageous for downsizing and weight reduction of the battery cell, and in addition to lithium unevenly distributed in the world, zinc is a material It can be said that it is far superior to lithium ion batteries in terms of supply and cost.
However, air zinc batteries are difficult to use as rechargeable secondary batteries because dendritic crystals (dendrites) are precipitated on the zinc electrode when charging and discharging are repeated, and are disposable for use in hearing aid or film camera exposure meters Has been put into practical use as a primary battery on the premise.

A research team such as Professor Yang Cheng of Sydney University in Australia succeeded in developing a new catalyst that can maintain chemistry stability even when charging and discharging air zinc batteries repeatedly. The catalyst developed by Professor Chen et al. Is made up of commonly available metals such as iron, cobalt, and nickel, which are readily available, and without using any rare metals such as lithium ion batteries, it is possible to use a rechargeable battery with high energy density Is realized.
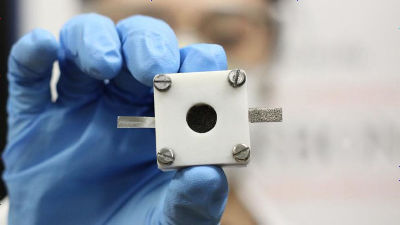
Conventionally, expensive metals such as platinum and iridium oxide were used as catalysts for air zinc secondary batteries, whereas by changing the three parameters of composition, size, and crystallization, it is commonplace for low cost We have succeeded in making catalyst from metal. It is expected to make inexpensive secondary batteries with high energy density including zinc as the main material of batteries.
In addition, Professor Chen's research team has confirmed that the battery capacity has only declined by 10% even after repeating charging and discharging for 120 hours 60 times. From now on, further improvements will be expected to realize air zinc batteries that can supply stable substitutable lithium ion batteries at a lower cost.
Related Posts: