A shipping stop order comes down to Google's genetic test kit "23 and Me"

ByNosha
The US Food and Drug Administration (FDA)Is to sell gene test kits "23 and Me"We ordered to stop selling the gene test kit.
FDA Inspections, Compliance, Enforcement, and Criminal Investigations 2013> 23 and Me, Inc. 11/22/13
http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm376296.htm
FDA Tells Google-Backed 23 and Me to Halt DNA Test Service - Bloomberg
http://www.bloomberg.com/news/2013-11-25/fda-tells-google-backed-23andme-to-halt-dna-test-service.html
My deadly disease was just a bug - mnt.mn
http://mntmn.com/pages/23andme.html
23andMe is a company that provides "Personal Genome Service (PGS)", a service that enables early detection of the incidence of specific diseases such as intractable diseases by analyzing genetic information, and co-founder of GoogleSergei · BrinMr. Ann Wodziski, a wife of Mr. Warziski, is a co-founder and a venture company founded in 2006 after being funded by Google.
The PGS provided by 23 and Me will send a genetic testing kit to the user's home, the user collects saliva and returns the kit after returning the kit, the examination is carried out and notification is given by e-mail after completion. Users can log in to a dedicated website and check results online, cost is 200 dollars (In Japan it is 19,800 yen) Has become. The user's genetic information is recorded in a database and used as a DNA sample for gene research.
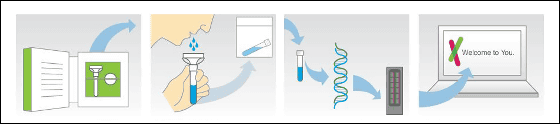
The genetic items that can be examined by 23andMe's test kit are limb girdle typeMuscular dystrophyYaGlycogen storage disease50 types of carrier status (Carrier Status) of intractable diseases such as caffeine metabolism (type I and type II)Hepatitis C(Drug Response) such as response to treatment of 50 items in total,AlcoholismYaAlzheimer's disease120 items of disease risk (Disease Risk) such as,LeprosySensitivity andMale pattern baldnessThere are a total of 254 items (Traits) including 60 items in total, of which 123 items are targeted by East Asian residents including Japanese. The price of about 161 yen per item of 23andMePGS is about 1 / 50th of the conventional genetic testing service.
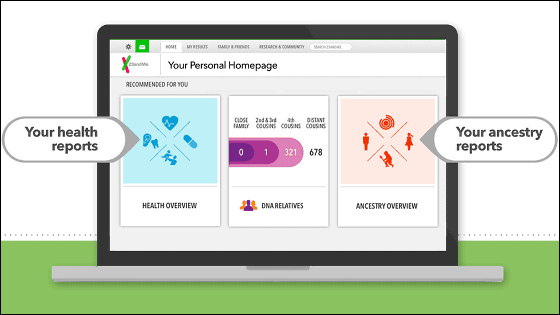
23andMe's PGS that can be easily and cheaply genetically tested has gained popularity in the United States and has been rapidly spreading due to TV commercials being flowed out but the FDA announced that on November 22, I ordered to stop selling the kit. The reason for the stop order is that Pandora's 23Ge mega does not meet safety standards stipulated by the FDA.
23andMe and FDA have held more than 14 direct consultations since 2009 and have handled hundreds of e-mail negotiations, in the course of which 23andMe submitted a test kit for investigation twice. However, as a result of checking the company's test kit, the FDA has caused false positive reactions in potential risk assessments such as ralphalin sensitivity, clopidogrel efficacy, fluorouracil toxicity, etc., causing unnecessary anxiety to the user, and conversely We concluded that there was a possibility that the opportunity to receive early treatment would be lost by false negative reaction and concluded that there is a problem with detection ability of 23 and Me's test kit.
In addition, there is a possibility that the user of the examination kit will conduct genetic testing on his / her own without receiving checks by doctors with specialized knowledge, and the possibility that health damage will occur or even if correct examination is done, It also points out the possibility that health damage may occur due to the fact that users can not fully understand.
ByBrendan Lim
There are also people who insist that they actually presented the result that using 23 and Me's PGS incorrectly raises the risk of incurable disease incidence.SpacedeckThe co-founder and chief technology officer, Lucas Hartman, pointed out that, in November 2010, when using 23 and Me's PGS, he possessed a limb zone muscular dystrophy (LGMD) gene carrier. Although he was disappointed with the result of this examination, he fully utilized the capability of self-proclaimed "net geek", ran around all the sites related to limb-band muscular dystrophy, further read the document on genetic engineering,PrometheaseAnalysis of the raw data of the gene provided by 23 and Me using the genetic analysis tool named "23" and "Me" revealed that there is a mistake in the algorithm of 23 and Me and it is not possible to express LGMD only under extremely rare conditions, It was judged that there was a risk of developing the disease. Hartman pointed out this, and in a few days it means that 23andme acknowledged an algorithmic bug and apologized.
In response to the FDA's suspension order to discontinue the test kit, 23andMe is aware of the importance of the relationship with the FDA and plans to promptly address the standards specified by the FDA. While it is estimated that easy genetic testing services will grow to a market size of $ 25 billion (about 2.5 trillion yen) within 10 years, improvements in inspection quality and reliability are the immediate tasks .
Related Posts:
in Note, Web Service, Hardware, Science, Posted by darkhorse_log