Heavy water, which is also used in nuclear reactors, turns out to be 'sweeter' than normal water

Water composed of
Sweet taste of heavy water | Communications Biology
https://www.nature.com/articles/s42003-021-01964-y
Since deuterium, which constitutes deuterium, has almost the same chemical properties as ordinary hydrogen, it is known that the chemical properties such as boiling point, melting point, and pH of deuterium are almost the same as those of ordinary water. However, according to the research team, discussions have been held since the 1930s, such as 'heavy water is sweeter than normal water.' Therefore, the research team investigated the cause of heavy water feeling sweet by letting humans and mice drink heavy water.
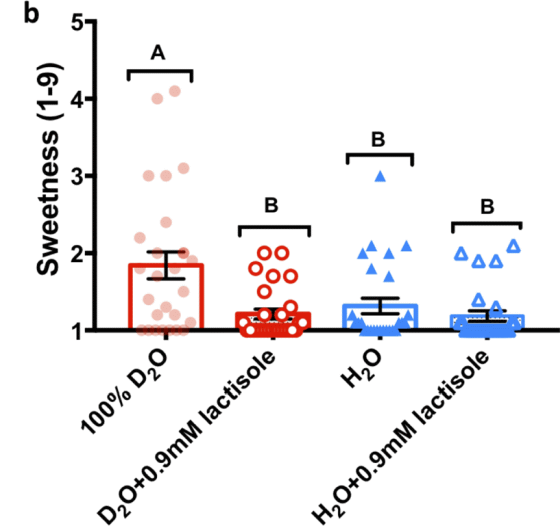
First, the research team prepared samples of sweeteners, umami ingredients, and bitter ingredients dissolved in heavy water and water at several concentrations to see if heavy water really felt sweet, and gave multiple subjects a sweetness level of 1-9. It was evaluated on a 9-point scale.
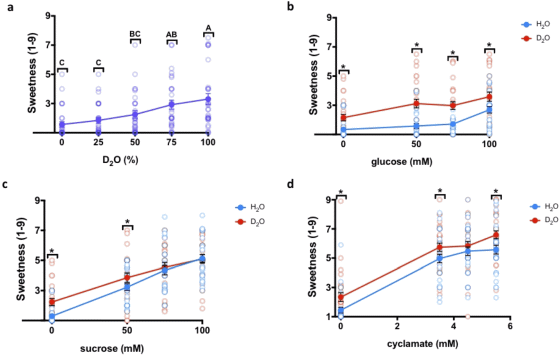
As a result, it became clear that when sweeteners such as 'glucose (b)', 'sucrose (c)' and ' cyclamate (d)' were dissolved in heavy water, the sweetness was stronger than when they were dissolved in water. It was. In addition, as a result of mixing heavy water with normal water (a), it was found that the greater the amount of heavy water mixed, the stronger the sweetness.
Next, 25 subjects
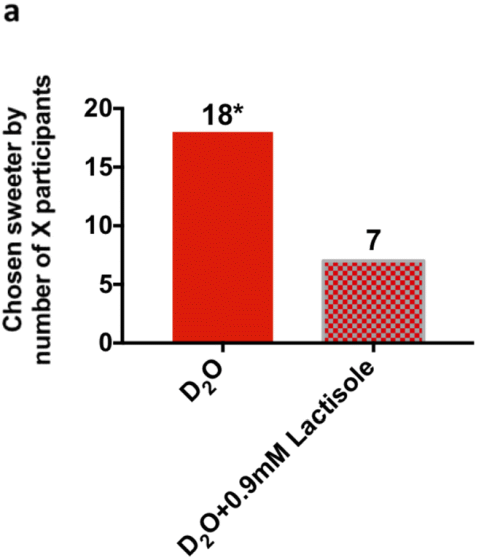
Furthermore, when the sweetness of four types of samples, 'heavy water', 'heavy water mixed with lactisole', 'water', and 'water mixed with lactisole' was evaluated on a nine-point scale, 'heavy water' was higher than the other three types of samples. It became clear that it also felt sweet. From these experimental results, the research team concluded that 'the human tongue has receptors that make heavy water sweet.'
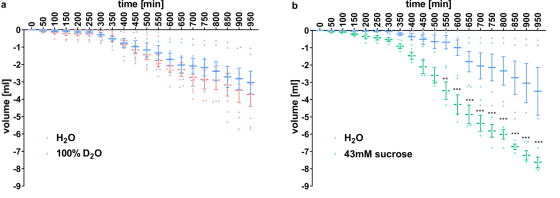
To investigate whether the tongue of mice also has receptors that make heavy water sweet, the research team put the mice in a breeding box that can drink two kinds of liquids, and the time when the mice drank each liquid Was measured.

As a result, there was no significant difference in the time to drink the two liquids when water and heavy water were added to the breeding box (a), and when water and 'sucrose-dissolved water' were added (b), there was no significant difference. It was observed that the time to drink 'water in which sucrose was dissolved' was prolonged. From this, the research team speculated that 'mice feel sucrose sweet, but heavy water does not.'
From the above results, the research team hypothesized that 'mouse sweet receptors do not feel heavy water sweet, and human sweet receptors feel heavy water sweet', and the human sweet receptors 'TAS1R2' and 'TAS1R3' Create a molecular model. As a result, it became clear that heavy water is slightly easier to combine with 'TAS1R2 / TAS1R3' than water.
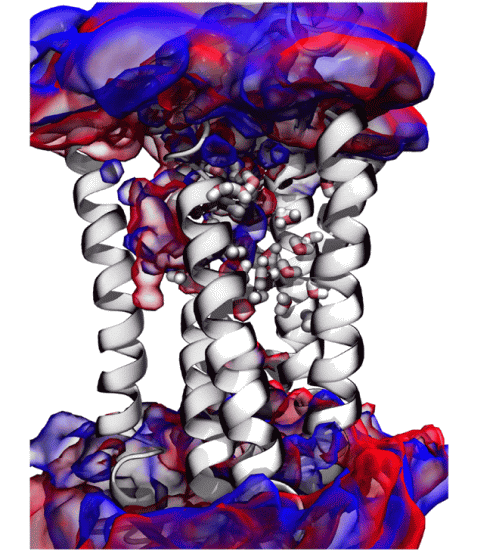
According to the research team, it was not possible to clarify the details of why heavy water feels sweet. However, he said, 'If we know the detailed structure of' TAS1R2 / TAS1R3 ', it will be clear why heavy water feels sweet.' I'm getting it.
Related Posts: