Is 'fire' solid or liquid or gas?

by Guido Jansen
Chemically, substances are classified into three types, solids, liquids and gases, but if it is said to classify a swinging fire as one of these three, you should be troubled by the answer. What on earth is fire? It is explained by animation by TED-Ed in an easy-to-understand manner.
Is fire a solid, a liquid, or a gas? - Elizabeth Cox - YouTube
When you sit in front of a flame, you should be able to obtain various sensations such as warmth, a fragrant wood burning, a crackling sound.

The flame in front of us changes shape without stopping, twisting or blinking, but what kind of substance is this "fire"? That's the theme of this movie.

Flame is not solid ... ...

It is not liquid either.

It seems like a gas shaking, but it is not a gas either. Scientifically speaking, the gas keeps the same state forever, but the flame is burned out eventually.

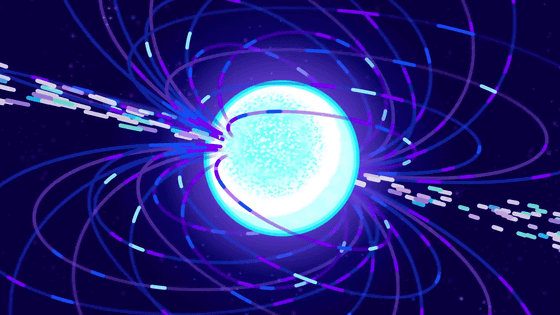
Also, although it is said that fire is the fourth state " plasma " following solid, liquid and gas, this is a mistake.

Unlike the other three, the plasma is not constant on the earth.

Plasma is made when the gas is exposed to a magnetic field, when it is overheated thousands of degrees, tens of thousands of degrees.

On the other hand, fire occurs at several hundred degrees, when paper or trees are burned at a temperature much lower than plasma generation.
If it is not solid, liquid, gas, or plasma, what is it like fire ...?

First of all, it is not "substance". Fire is a sensory experience of a chemical reaction called "burning". This leaves the leaves turn red in autumn ... ...

The apple turns spoiled and discolored ...

The same thing as fireflies glow. The difference between these and the flame is to appeal to many 'senses' at the same time, while generating intense experience from physical objects.

Burning uses fuel, heat, oxygen, etc. to create sensory experiences.

When the log reaches the ignition point at the camp fire, the cell wall is decomposed and sugar and other molecules are released into the air.

This molecule reacts with oxygen in the air, producing carbon dioxide and water.

At the same time, the water remaining in the log evaporates, expands, bursts the tree and makes a crackle.

It is because the gravity gives the feature of "tapering down" to the flames that flames are burning towards the sky.

Without gravity, dispersion due to the concentration of molecules does not occur, so the flame under zero gravity becomes completely different from the earth.

And these phenomena can be confirmed because fire creates "light".

Molecules emit light when heated, but the color of the fire depends on the temperature of the molecule, and changes to white or blue when the temperature is high.
Also, the type of molecule contained in the fire also affects the color.
Carbon molecules that are not chemically reacted in logs are smeared in the form of soot and produce yellow or orange light.

Meanwhile, metals such as copper, calcium chloride and potassium produce different flame colors such as blue and pink.

Also, although the fire creates heat, this heat keeps the fuel above the ignition point and allows the fire to continue to burn.
And no matter how big a flame it will eventually run out of fuel and oxygen, it disappears and disappears.

Related Posts: