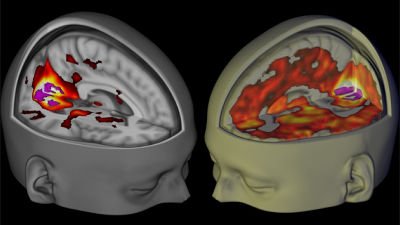
Illegal drug MDMA known as ecstasy is designated as FDA as "landmark treatment"

ByFreestocks.org
A method known as "ecstasy" and using MDMA designated as an illegal drug for PTSD treatmentAmerican Food and Drug Administration(FDA) "Breakthrough therapy(Breakthrough treatment) "was specified. If this therapy is approved, research that uses PSD and magic mushrooms for treatment can also make a big step forward.
MAPS - FDA Grants Breakthrough Therapy Design for MDMA - Assisted Psychotherapy for PTSD, Agrees on Special Protocol Assessment for Phase 3 Trials
http://www.maps.org/news/media/6786-press-release-fda-grants-mdma-breakthrough-therapy-designation-for-mdma-assisted-psychotherapy-for-ptsd,-agrees-on-special-protocol-assessment-for-phase-3-trials
All clear for the decisive trial of ecstasy in PTSD patients | Science | AAAS
http://www.sciencemag.org/news/2017/08/all-clear-decisive-trial-ecstasy-ptsd-patients
Ecstasy could be 'breakthrough' therapy for soldiers, others suffering from PTSD - The Washington Post
https://www.washingtonpost.com/national/health-science/ecstasy-could-be-mdma-breakthrough-therapy-for-soldiers-others-suffering-from-ptsd/2017/08/26/009314ca-842f-11e7-b359-15a3617c767b_story.html
3,4-methylenedioxymethamphetamine(MDMA) is known as ecstasy and is a drug that affects neurotransmitters in the brain. When ingestion happiness, sociability and empathy are improved and even if you do not sleep, you become uncomfortable, but there are side effects such as severe medication sickness. Read the following article to get a good idea about the efficacy of MDMA.
Movie that understands the influence and side effects of MDMA popular with young people as a party drug on the brain - GIGAZINE
Although MDMA is regulated by law in many countries as being harmful to the human body, attempts have been made in each country to adjust usage and dosage and use it for therapeutic purposes, and in 2017 aiming for alcoholism treatment We started clinical trials.
The world's first clinical trial using a party drug "MDMA" for alcoholism treatment started - GIGAZINE

According to the nonprofit organization "Magazine Interdisciplinary Research Society (MAPS)" published, the treatment using MDMA was designated as FDA as "breakthrough therapy (landmark treatment)". The study of MAPS consists of three sessions, and in the clinical trial at the phase 2 stage, MDMA removes the fear of the subjects, regains the power of introspection and communication, improves sympathy and the same capacity It was reported that it succeeded. In the future, FDA will be closely involved with MAPS and we plan to complete Phase 3 clinical trials as efficiently as possible.
Breakthrough therapy was enacted in 2012 as a designation system for certain treatments to be developed and reviewed more quickly than other treatments, and one or more to be designated as breakthrough therapy In several clinically important endpoints, it is necessary for suggestive clinical evidence to suggest improvements over existing treatments. Up to now, about 200 medicines have been designated for breakthrough therapy.
If it is proven by MDAP that MDMA is useful for treatment, there is a possibility that other studies trying to use medicines such as LSD and Shiroshin prohibited by law as being "harmful to the human body" for medicine will also advance It is seen as there is. Psychotropic drugs are being studied in various countries around the world, but it is also a fact that studies can not be promoted easily due to the barriers of law. In the past LSD research conducted at Imperial College London, it is necessary to conduct research from the Ethics CommitteeIt took nine months to obtain permissionAnd that. Researcher David Nut said about the announcement of MAPS "This is not a major advance in science." "It is an obvious fact over these past 40 years that these drugs are drugs, a big step in" acceptance " It is. "

ByJanine
He is the founder of MAPSRick DublinSince he was convinced of the potential possibilities as a medicine for MDMA, he established MAPS as soon as the Drug Enforcement Administration designated MDMA as an illegal drug in 1986. And has spent millions of dollars on research using MDMA as a treatment for PTSD and addiction. A paper that claims that MDMA will be useful for treatment for the first time in the United States has been announced in 2011 and since then, a large-scale clinical trial of Phase 2 in which MAPS has invested in many countries such as the United States, Canada, Israel, etc. has been conducted . Although the results of these clinical trials have not been announced, they have received good evaluation by the FDA. In this clinical trial we treated 107 subjects who had suffered from PTSD on average for 17 to 18 years and found that 61 of the 90 subjects who could continue their research for 12 months, He seems to have suffered from PTSD.
For example, John Lubeckie, who suffered from PTSD after returning from Iraq as a soldier, heard that he heard the sound of a helicopter or bombardment in a place where there was nothing and could not sleep at night and drank alcohol until he fainted Thing. Although he did not improve symptoms at the facility for veterans, he tried to commit suicide five times, but finally taking MDMA capsules to participate in MAPS clinical trials, suffering It seems that it was released from.

However, there is a possibility that pure MDMA used in treatment and ecstasy as sold as a street are different things, some ecstasy on the market may contain substances harmful to the human body The house points out. Also, MDMA of the type used for treatment also has side effects, the body becomes overheated, stress hormoneCortisolIncrease the amount of secretion, symptoms such as anxiety, memory disorder and so on. For these reasons, some researchers oppose attempts to use MDMA to treat PTSD.
The phase 3 clinical trial conducted by MAPS is targeted at 200 to 300 subjects, and the cash flow is a problem at the moment. The target amount for conducting clinical trials is $ 25 million (about 2.7 billion yen), but there are still less than 12.5 million dollars (about 1.35 billion yen) of funds. If fundraising is completed, clinical trials can be started in 2018 and scheduled to end in 2021.
Related Posts:
in Science, Posted by darkhorse_log