Five things that I do not know well about water

ByJanet Ramsden
"Water" necessary for living beings to live is surprisingly small as opportunities to think about water carefully because of being too familiar. However, as for the water that everyone feels natural, it is as follows when summarizing the representative five things which have not been elucidated at all.
Five Things We Still Do not Know About Water - Richard Saykally Takes Us Inside Waters' Mysteries
http://nautil.us/issue/25/water/five-things-we-still-dont-know-about-water
◆ 1: There are many kinds of "ice"
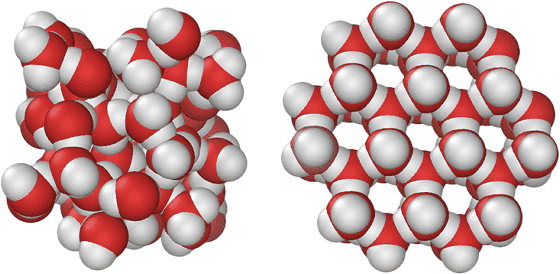
Where everyone knows that water will "ice" when it freezes. It is known that ice, which is solid water like this, is quite unusual, and its volume increases more than liquid state. this isHydrogen bondingIt is known that water is the smallest volume (= high density) when it is 4 degrees Celsius (hereinafter all temperatures are expressed in degrees Celsius).
However, it is not taught at school that "ice of solid state of water is not one kind", there are many people who do not know. At present, there are 17 types of ice even by being known, and in addition to "Ih" which we generally call ice, there are very small amounts of "Ic" in the natural state, and high pressure Fifteen kinds of ice that can exist only in the lower universe have been found.
Until the limit of the closest packing structure is reached by applying pressure from the "ordinary" ice condition (right) with well-formed hydrogen bonds and low density,Ice takes various shapesIt is thought that, in the future, ice of a state other than the known 17 kinds may be discovered from now on.
◆ 2: Water evaporation mechanism
The change of a substance from a liquid to a gas is called "vaporization (evaporation)", and it is also due to vaporization that water evaporates into water vapor. The rate of evaporation of this water has a great influence on the generation of clouds in the atmosphere and it is one of the very important factors for predicting weather.

ByMervi Eskelinen
However, the mechanism by which water evaporates is still not fully understood. If the salt is added to water, the surface tension rises and the surface tension wave is suppressed, the evaporation rate should theoretically decrease, but the evaporation rate has not been elucidated in the experiment, for example, almost no change in evaporation rate is seen in the experiment Thing.
◆ 3: Is the surface of water acidic or basic?
It is known that the fog that Niagara Falls creates contains negative ions. Not only Niagara Falls but also many waterfalls are emitting negative ions because hydroxide ions are accumulating on the surface of water, the pH of the liquid surface is considered to be more basic than 7 I have done it.

ByMATAVI @
However, in recent experiments and simulations, it is known that the surface of liquid water actually contains more hydrogen ions than hydroxide ions, and the pH is less than 7, that is, it is acidic.
◆ 4: Amorphous ice
As the temperature decreases, water becomes solid ice at less than 0 degrees, but when cooled slowly, it does not freeze even if it is below 0 degreesOvercoolingThe phenomenon occurs. It is also known that coagulation will occur at -38 degrees no matter how slowly cooling it is.
However, if water is cooled slowly, there is no regularity in the binding of molecules in the low temperature region below -38 degreesamorphousA state of "Amorphous iceIt is known to be able to take crystals called.
The solid has a regularity in the molecular structure, and the liquid generally has no regularity in the molecular structure. In the case of water, however, in the case of water, a special state . In other words, amorphous ice is "solid" because it is solid and has no fluidity, but it is the same as "liquid" in that there is no regularity in the molecular structure, and it can be said that it is "hard but liquid" like glass at extremely low temperature That's why.

There is a dispute as to whether the glass is in a liquid state, a solid state or an independent glass state,Kyoto University's research that "glass is not a liquid" overturning the conventional theoryHas also been announced. Therefore, it seems that opinion will be divided as to whether amorphous ice is solid or liquid or another state.
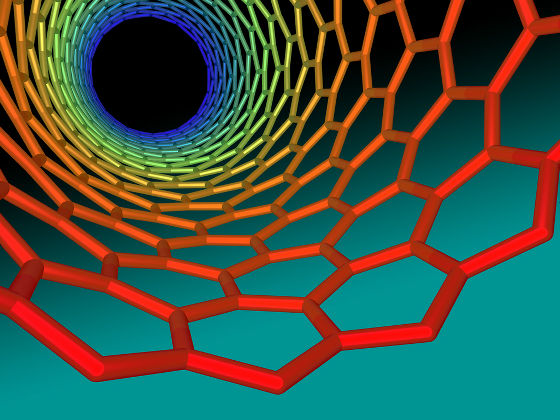
◆ 5: Quantum size water
Since it became possible to produce carbon nanotubes and proton exchange membranes, the challenge of confining water molecules in the quantum size has been advanced. It is known that quantum effects can be demonstrated by confining water at hundreds of molecules level and extremely small amount in a narrow space both experimentally and computationally.
ByGeoff Hutchison
It is thought that the characteristics of water showing these quantum effects influence every area from biology to geology, for example, it is expected that a freshwater device can be designed to more effectively produce pure water from seawater It is being done. However, at the present time, even trapping water is not going well, and studies in this field have a lot of problems to be solved.
Related Posts:
in Science, Posted by darkhorse_log