Lithium-ion battery using nanocrystal with power doubled compared to the conventional one will be developed
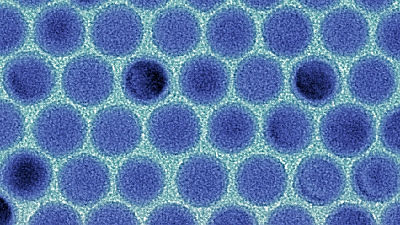
Lithium-ion battery can store a lot of energy despite its relatively compact size and lightweight size, so it is used for various things from mobile phones to cars,Zurich Institute of TechnologyMaksym Kovalenko's research team used nanocrystals as electrodes and developed a lithium-ion battery that is almost twice as powerful as before.
Tin nanocrystals for the battery of the future
http://www.ethlife.ethz.ch/archive_articles/130408_li_ionen_fb/index_EN
When charging the lithium ion battery, lithium ions in the anode are absorbed into the cathode and emitted from the cathode when discharged. During discharge, negatively charged electrons move through the external circuit to the anode, but in order to keep the charge balance, positively charged lithium atoms pass through the electrolyte in the battery. Is the basic mechanism of lithium ion battery. At this time, most of the batteries on the market are made from an oxide of nickel or cobalt / manganese as the anode, and graphite as the cathode.
However, in this study, we studied a mechanism to store twice as much energy as conventional by creating electrodes with tin crystals instead of nickel. Tin can absorb a minimum of 4 lithium ion atoms, but since it has the property that its volume increases up to 4 times due to the absorption effect and it shrinks at the end of the discharge, researchers can solve this problem I had to solve it.

So, when the research team created extremely small crystals and embedded them in permeable, conductive carbon substrates, they succeeded in creating substances capable of absorbing and releasing a large amount of ions. The electrode made by this method absorbs lithium ions so that the sponge absorbs water and releases ions at the time of discharge. If the electrode was made with only small tin, it was impossible for such a thing. At this time, it is possible to control the crystal size by adopting the method of choosing the tin crystal as small as possible and growing it later.
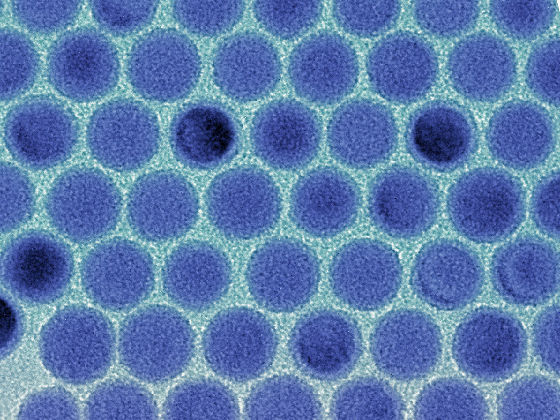
Experiments revealed that 10 nanometer crystals are the most efficient, and finally it has become possible to store twice as much energy in the battery with uniform nanocrystals and carbon and binder. In the future, discovery of carbon substrates and binding agents that work most efficiently is attracting attention in order to lower production cost and ensure stable supply.
Related Posts:
in Science, Posted by darkhorse_log